Voglibose, an alpha-glucosidase inhibitor, is effectively used for diabetes treatment. It delays glucose absorption at the intestinal level and thereby prevents a sudden surge of glucose after a meal. It was developed in 1994 in Japan by Takeda Pharmaceuticals Industries Ltd. And shows high activity so small doses can effectively prevent side effects in the gastrointestinal tract.
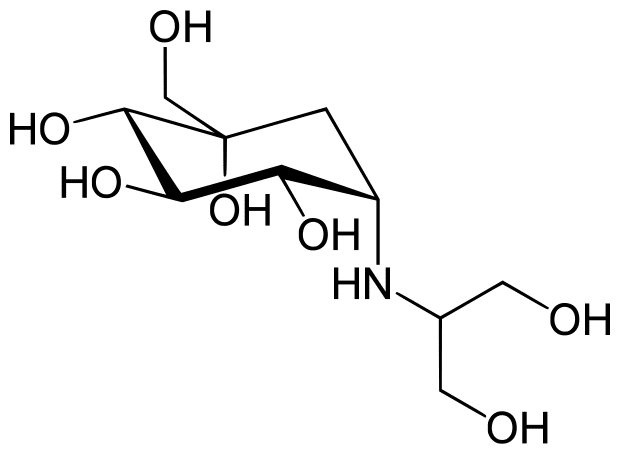
The absence of a chromophore or conjugation in the molecule makes it difficult to detect by spectroscopic absorption in the UV – Visible region. However, post-column derivatization with a fluorescence agent provides a high sensitivity and selectivity for Voglibose in pharmaceutical formulations. This method has also been notified in Japanese Pharmacopoeia 16th edition as well as Indian Pharmacopoeia 2014.
We have introduced a post-column fluorescence derivatization system and the test conditions employed are
- Column- SS amino column 250mmX4.6mmX5μ
- Column temperature- [latex]25^0[/latex]C.
- Mobile phase-37:63 mixture of phosphate buffer at ph 6.5 and acetonitrile
- Flow rate – 0.6ml/min
- Reaction coil temperature- [latex]100^0[/latex]C
- Detector- Fluorescence with excitation at 350nm and excitation at 430nm
- Fluorescence agent-Taurine 6.25 g +2.56 g Sodium periodate in 1000ml water
The method provides high precision and sensitivity of detection.
















