
The test for particulate contamination in parenteral preparations is a critical test and is a mandatory pharmacopoeial requirement. The pharmacopeias prescribe either a microscopic particle count or a light obscuration liquid particle counter, to carry out this test. We at Arbro have been offering the microscopic particle count test for many years now and are pleased to share that we have recently installed the light obscuration of a liquid particle counter in our laboratory, to fulfill this frequent requirement from our customers involved in the manufacturing of parenteral preparations.
What is particulate contamination?
The pharmacopeia defines particulate contamination as “the unintentional presence in injections and infusions, of extraneous, mobile, un-dissolved substances, other than gas bubbles. These solids may consist of individual components or a mixture of cellulose, glass, rubber cores from vials and plastic fragments”
What is a potential source of particulate contamination?
Even though sterile manufacturing facilities and processes are designed and controlled to minimize the generation of particulate matter. Still, it is possible for contamination from any of the following sources to enter the product –
- Manufacturing environment
- Process equipment
- Manufacturing personal
- The packaging components
- Can be generated by chemical reaction or incompatibility of ingredients of the formulation.
Why should it be controlled?
The test for particulate matter primarily deals with non-infectious particles which may be present in an intravenous formulation. Though these particles are not infectious, it is known that particles larger than 5 µm can damage or even block blood vessels leading to severe adverse effects. Particle contamination can even lead to embolisms in the brain and respiratory system which can be fatal.
What are the Pharmacopoeial requirements?
Solutions for injection administered by the intramuscular or subcutaneous route must meet the requirement of particle matter in the injection. Parenteral preparation including solution constituted from sterile solids is expected to be free from particles of approximately 50µm or more that can be observed by inspection with the unaided eye. However, Parenteral preparations in containing 100 ml or more of a single dose large volume injection intended for administration by intravenous infusion should comply with the limits of subvisible particle prescribed in this test.
The test does not apply to multiple dose injections, to single dose small volume parenteral preparations and to parenteral solutions constituted from sterile solids.

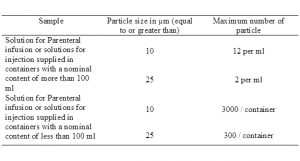
What are the prescribed methods?
- Light Obscuration Particle Count Test
- Microscopic Particle Count Test
- Visual inspection
What is the principle behind the light obscuration liquid-borne particle counter?
The liquid particle counter operates on the principle that the light extinguished by a particle in a liquid within a laser beam is a direct function of its area. Particles obscure the laser beam during transit through the laser beam. The pulses produced by electronically detecting the total laser light minus the light obscured by the particle are used to size the particle. These pulses are measured by an analog to digital converter in the sensor. The liquid is presented to the optical system through a rectangular capillary. The capillary has a window attached to both the front and back sides which are coated to reduce reflections. A central software application controls the hardware, analyzes the data and stores the data future interpretation. The variation in light caused by the passing of a particle is electronically detected by the photodetector. This signal is then amplified and converted to its digital equivalent. The value of this digital signal is converted into an equivalent particle size in a microprocessor. The different size particles are counted and stored and made available for transmission to the data display system upon request.
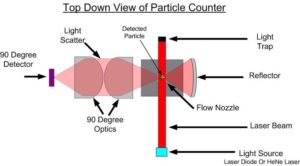
The test is carried out under conditions limiting particulate matter, preferably in a laminar-flow cabinet.
Why APSS 2000 is the system of choice?
The APSS 2000 particle measurement system offers several advantages, some of these are listed below-
- The system measures 100% sample volume by passing it through the measurement orifice for greater accuracy.
- The integrated syringe sampler has a procession of 0.1 milliliter producing repeatable results
- the software provided with the system comes with the comprehensive software development documentation and full IQ, OQ, and PQ protocols and complies with FDA 21CFR-11
- Methods can be built and stored, thus reducing errors for routine analysis as per the USP, EP, or JP standards
- Automated particle counter calibration wizard for full calibration or routine verification
- Alarm levels for pass/fail criteria ensure quality control
- Particle measurement results can be obtained both in terms of number of particles per milliliter or number of particles per container
What are the applications for the liquid-borne particle counter?
Despite the most obvious application of pharmaceutical parenteral monitoring to USP, EP, JP, and FDA standards, the system can also be used for the following –
- Parts/medical device cleanliness testing
- Laboratory water sampling
- WFI and Purified Water testing
- Filter efficiency testing
The system has been installed and duly qualified in our laboratory. If you wish to avail the service or need any further information about this, please feel free to contact us using the quick query form on the right or call us now on +91-11-45754575!
To see other new addition to our lab you can visit the New Equipment Page by clicking here!
















