Ion chromatography has nowadays made its place in several fields, including pharmaceuticals, foods & beverages, water analysis, etc.
But despite being a widely used technique, there are people who do not know much about ion chromatography analysis. If you are one of them, this article will help you out.
Here we will discuss everything you need to know about this method, ranging from what it is, its principles, and its popular applications. So you will know how it works in different domains.
What Is Ion Chromatography?
Ion chromatography or Ion exchange chromatography is a branch of High-Performance Liquid Chromatography or HPLC. It has a potential of separating charged molecular species present in the mobile phase stream.
The charge bearing molecules are separated from non-ionic species due to their affinity for oppositely charged sites on the ion exchange stationary phase packed inside the column.
The scope of applications of ion exchange chromatography extends from the separation of inorganic cations and anions to organic entities such as amino acids, peptides, proteins, and nucleosides.
In simpler words, you can also say that it is a method used for separating any compound’s ions on the basis of their interaction with stationary (resin) and mobile (eluent) phase. The process consists of two columns named anion column and cation column that attract anions and cations, respectively. By separating these ionic species, one can measure their concentrations in the given substance.
Ion exchange chromatography had its origins in 1850 when Thompson applied it to study the adsorption of ammonium ions on soils. In 1947 Spedding and Powell used the technique for separation of rare earth.
The concept was extended in 1950’s by Krauss and Nelson for separation of common anions such as chlorides, fluorides, nitrates, and sulfates. The technique proved to be a boon for separation and identification of anions and cations present in several complex matrices and simultaneously conductivity detection proved to be an ideal mode for quantification of isolated species in the 70’s decade.
Today the applications of the technique have grown due to the availability of a host of ion exchangers, improved sensitivity of detection and a multifold improvement in speed of analysis. The present article discusses briefly some prominent areas of application where the technique is in wide use. However, before proceeding to applications a brief review of principles of the technique should be useful.
Basic Principles
Electrostatic interactions of ionic molecules with opposite charges present in the stationary phase is the basis of separation by ion chromatography. The ions or charged molecules that have a lower affinity or do not bind at all are eluted first followed sequentially by species that have stronger interactions.
The binding force can be altered by changing the composition or pH of the mobile phase. In general, positively charged molecules or ions bind to anion exchangers and negatively charged entities bind to cation exchangers.
Ion-exchange columns comprise functional groups such as weak/strong acids or weak/ strong bases. Special resins with amphoteric features also exist.
Quaternary ammonium group has anion exchange capabilities whereas sulphonic acid group acts as a cation exchanger. Such functional groups are capable of maintaining their charges over almost the complete pH range.
Detectors used in Ion chromatography
Conductivity Detectors
Conductivity detection is the main detection technique applied to ion exchange chromatography. It offers a universal detection mode for charged species present in the mobile phase.
The conductivity of the mobile phase is dependent on ionic strength, types of ions present and the temperature of the medium. The sensitivity of detection will depend largely on the difference between conductivities of ionized species present and the composition of the mobile phase
Electrochemical Detection
Application of an electric potential between the plates of the electrode can lead to reactions based on oxidation or reduction of the species present. In amperometric mode a fixed potential is applied to a working electrode with reference to a reference electrode and the current developed as a result of a redox reaction is measured as a signal. In voltammetric detection a varying potential is applied to a working electrode with respect to a reference electrode and the resulting current is recorded.
Spectroscopic Methods
Spectroscopic methods are used indirectly for conducting ions do not absorb significantly in both UV- visible and fluorescence regions. Use of selective chelates and ion pairing agents can assist detection in such modes.
Similarly, direct detection is difficult with reflective index detectors and indirect methods can be applied.
Hyphenated Techniques
In recent years ion pair chromatography has been successfully adapted to hyphenated techniques such as ICP- MS for ultra-trace level estimations of cationic species as well as in speciation studies.
Major Application Areas
Water analysis
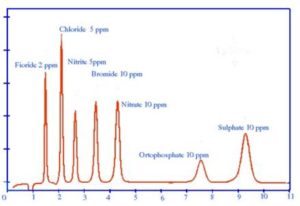
Ion chromatography with conductivity detector has found wide-scale applications for monitoring of anion concentrations such as chloride, fluoride, bromide, nitrates, nitrite, fluoride, chlorate and bromate in drinking water from different sources.
Use of conductivity detection followed by post-column reactions has supported UV-based detection for low levels of such anions together with the low-level determination of oxyhalides such as bromates, chlorites, and chlorates which are present in several times lower concentrations in comparison with other common anions.
Pharmaceuticals
Ion chromatography has over the years matured as an analytical technique for determination of the active pharmaceutical ingredients, excipients, products of degradation and impurity profiling of formulations.
In addition to the analysis of finished products, the technique has also been applied for quality control of raw materials, intermediates, validation of blender cleaning solutions and effluent streams. The technique shows promise as it is sensitive to some compounds that show little or no absorbance in spectroscopic determinations.
Foods and Beverages
Ion Chromatography finds several applications in Food and beverage industries as it provides an opportunity for conducting analysis with little or no sample pretreatment and a possibility of estimation of a range of analytes in a single run.
Some common applications using conductivity detection are:
Acids: lactic, citric, maleic, ascorbic, etc in drinks and beverages
Sugars: sucrose, fructose, lactose, glucose in alcoholic and non-alcoholic drinks
Anions and cations in waters and sweeteners: Ca, Mg, Sr, Ba, fluoride, bromide, fluoride, nitrate, phosphate and sulfite
Wines: Sulphites, nitrates, and polypropylene glycols
Certain applications may require far more sensitive detection such as mass spectrometry in selected ion monitoring mode. This, in particular, provides very low detection levels and differentiation between co-eluting compounds in complex food matrices.
An example is a detection of perchlorate in soils, vegetables, milk, and raw waters. Perchlorate determination has gained significance as it inhibits uptake of iodide by the thyroid gland. Similarly, carbohydrates such as polysaccharides can be detected at ultra-trace levels in beverages and honey using SIM Mass spectrometry.
Clinical Studies
Ion Chromatography has found applications in clinical diagnosis and research. It is possible to monitor trace levels of common ions such as sodium, potassium, ammonium, magnesium, calcium, fluoride, nitrate, phosphate and sulfate in biological specimens such as body tissue extract, blood serum, and cerebrospinal fluid in less than an hour.
The scope of uses of ion-exchange chromatography is increasing at an impressive pace, and due to its versatility, the technique is providing solutions to analysis that remained unsolved in the past.
Sample Collection in an Ion Exchange Chromatography Lab
Now that you have understood ion chromatography uses, you should know how professional labs collect different types of samples for conducting their experiments. The collection and preparation of samples in ion chromatography testing majorly depends on the kind of substance that has to be tested. Here is how different types of units are prepared:
Liquid Samples
Before conducting ion chromatography analysis on liquid substances, they have to be filtered out. This helps with the removal of any matter or sediments present in the sample. Plus, it reduces the possibility of microbial alteration prior to conducting the tests. Before collecting these samples, the following steps are followed:
- The syringe or bottle gets cleaned with the sample water three times.
- Then they are filtered through at least 0.45 um small filters.
- The same steps are followed for the collection vial (small bottle in which sample gets collected).
- Then the vial is filled up to its brim with the filtered sample. The quantity here should be a minimum of 5 mL.
Once done, the samples are finally stored in a cold place until they are taken out for processing and testing.
Solid Samples
The solid samples of ion chromatography are cleaned with water or cationic acids. This helps with the removal of any ions present on the solid’s surface, and then they are stored in cool. The minimum quantity specified here is 2 to 3 cm2. Professionals prefer the same process for organic liquids.
For any kind of substance, there is no maximum limit of sample usage. There is only a minimum amount of sample required for ion chromatography testing.
Ion Chromatography at Our Lab
We can help you with our state-of-the-art ion-exchange chromatography lab. You can check out the given uses of ion-exchange chromatography and see what kind of testing you require. Our experts will guide you with the entire associated process and testing.
You can reach us through the given ‘Quick Query’ form or call us on +91-11-45754575. We will be happy to assist you.
















