Quality in Food Chemistry PT Scheme Results for ARBRO
ARBRO Pharmaceuticals Ltd recently participated in the Quality in Food Chemistry PT Scheme (FC4083) organized by LGC Limited which is the UK based, international life sciences measurement and testing company. We are happy to report that our PT results were declared as satisfactory which is how the result is declared if proved correct.

A number of samples were dispatched to various participating laboratories for testing like
- An aqueous solution containing preservatives
- An aqueous solution containing sweeteners
- An aqueous solution containing artificial colors
- Green tea
- Flour
- Vegetable fat spread with olive oil
- Tomato paste
- Edible oil
Arbro testing lab undertook to detect the values of artificial colors in the aqueous solution sample and our results were declared as satisfactory on two counts while one was not assessed because of discrepancy in the assigned value derived by LGC.
We are happy to report that we had no ‘questionable’ or ‘unsatisfactory results’. We detected the values of artificial colors through the HPCL method and we were able to get the following values for the three colors
- Ponceau 4R – 28.1mg/L as compared to the assigned value of 25.8
- Sunset yellow – 64.1mg/L as compared to the assigned value of 61.1
- Indigo carmine – 67.7mg/L as compared to the assigned value of 70.7
Quite a large number of food chemistry proficiency tests are carried out annually across the world according to established protocols. ARBRO Analytical Division participates in these proficiency tests to ensure that our analysis of food chemistry is on the right track and it helps us monitor our own performance. When there is a consistency in our results as compared to the assigned value it works as a certificate that our food testing services are proficient and we are on par with the standard results.
Tetracyclines in Honey FAPAS PT Results
Our lab recently participated in an international proficiency testing programme number 02142 conducted by FAPAS®; The Food and Environment Research Agency; Sand Hutton, York, UK, YO41 1LZ. Our laboratory number was 32.
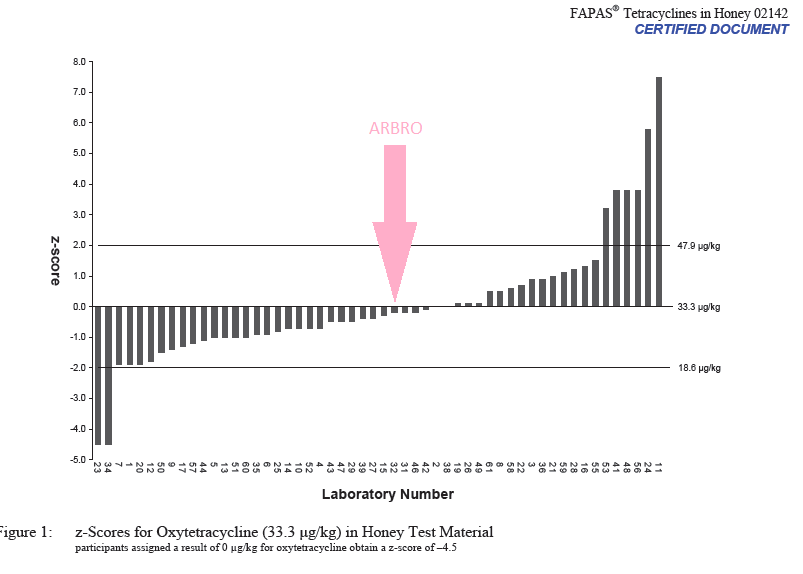
We have reported a level of 31.8 μ/kg for Oxytetracycline; the material had an assigned value of 33.3 μ/kg thus earning us a Z score of -0.2 which is well within the acceptable range of ±2. The materials were dispatched to 70 participating labs in 27 countries of which 61 submitted the results. Only 46 labs gave acceptable results within the ±2 limit for Z score and our lab was amongst the top 10 labs in terms of accuracy of the results.
Pesticides in Grape Puree FAPAS PT Results
Our lab recently participated in an international proficiency testing programme number 19103 conducted by FAPAS®; The Food and Environment Research Agency; Sand Hutton, York, UK, YO41 1LZ.
We have successfully detected all the pesticides present in the sample covered by our method of analysis. The materials were dispatched to 118 participating labs in 30 countries of which 104 submitted the results.
QMS PT Results for Salmonella in Skimmed Milk Powder
ARBRO Pharmaceuticals Limited recently participated in the Quality in Microbiology Proficiency Test Scheme (QMS – PT) MC6123 which was organized by LGC Limited which is the UK based, international life sciences measurement and testing company.
We are happy to report that our QMS – PT results were declared as satisfactory. Out of a number of laboratories that performed the lab test worldwide our testing laboratory was one of those that were able to carry out the proficiency test in a satisfactory manner. Our result had no questionable or unsatisfactory remarks.

Out of the various samples sent to the laboratories, ARBRO analytical Division received the sample 06D which was 25g of skimmed milk powder for detection of Salmonella Thompson Citrobacter freundii. Our testing lab was successfully able to detect the presence of Salmonella species in the sample. Our result showed a score of 94.6% which was declared by LGC as a satisfactory result.
Salmonella testing
The Salmonella species can cause severe foodborne illnesses by ingesting contaminated foods especially of animal origins like chicken, pork, eggs, and milk. Salmonella bacteria live in the intestinal tract of infected animals and humans and can be transmitted through the feces. Salmonella is cable growing in a wide range of temperatures from 6 – 46oC but optimum growth is in temperatures between 32 – 37°C.
The increasing demand for street foods and minimally processed, ready-to-eat products has also increased the need for food safety from microbial infections. Therefore microbiological testing of pathogens like salmonella becomes important as the food product must have salmonella contamination which is below the regulatory standards for that food product. Analyzing uncooked and raw foods is also necessary to ensure that food supply is safe and to prevent outbreaks of salmonella infections that can sometimes be fatal especially in infants, elderly and pregnant women.
ARBRO Results for PHARMASSURE Pharmaceutical PT Scheme
ARBRO Pharmaceuticals Limited recently participated in PHARMASSURE – Pharmaceutical PT Scheme PH0106 which was organized by LGC Limited which is a UK based international life sciences measurement and testing company.
The samples provided by LGC were of three types where the participating testing labs could choose to do basic chemical testing, advanced chemical testing and microbiological testing. The sample selected by ARBRO Pharmaceutical Ltd was in the advanced chemical testing category and in this round too we achieved satisfactory results as we had no unsatisfactory or questionable results.

ARBRO got samples of 1 x 0.1g lidocaine HCl reference standard (purity 100%), 1 x 0.3mL 2,6-dimethylaniline reference standard (purity 100%), 1 x 10mL sample A. Arbro testing lab sent the report four analytes as follows
- Lidocaine HCI for which we got a result of 0.505 against the assigned value of 0.506
- Retention time – Lidocaine HCI for which our result was 3.4 but the assigned value was not available
- 2,6-dimethylaniline for which we got a result of 0.0203 against the assigned value of 0.0200
- Retention time – 2,6- dimethylaniline for which we had a result of 14.9 but the assigned value was not available
Since assigned values were not available for two out of the four advanced chemical testing carried out by Arbro testing lab hence LGC could declare a satisfactory result for only two of the four tests we performed.
Pharmaceutical testing
With the advent of globalization innovations in prescription drugs and the launch of nutraceuticals and dietary supplements has given rise to the need to bring out new products quickly but safely. There is a requirement for world-class pharmaceutical testing to validate compliance with regulatory requirements.
ARBRO Pharmaceuticals Limited has a number of laboratories that specialize in a range of chemical and microbiological analysis. We deliver analytical solutions that can ensure the safety and effectiveness of raw materials as well as pharmaceutical products.
















